Short Answer
Referring to Figure 12-9, a substance carried from point A to B absorbs 50. J and finds its internal energy has increased by 20. J. Going from B to C the internal energy decreases by 5. Joules.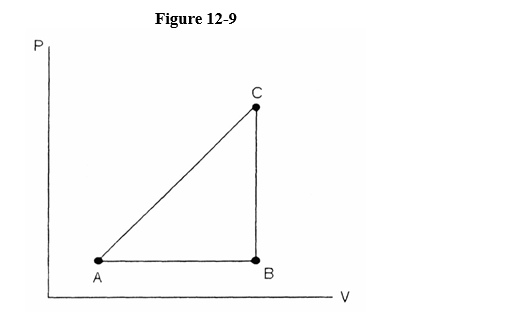
(a) How much work was done from A to B?
(b) How much heat was absorbed from B to C?
(c) How much work was done going from B to C?
Correct Answer:

Verified
(a) 30. J
...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q17: A cyclic process is carried out on
Q18: The Otto cycle has how many strokes
Q19: The work done by the gas is
Q20: The Department of Energy develops a new
Q21: A gas is confined to a rigid
Q23: Two processes are shown on the p-V
Q24: An example of a reversible process is
Q25: Why don't we use "efficiency" for rating
Q26: The change of the internal energy of
Q27: One of the most efficient engines built