Multiple Choice
The "set-up" for the problem "What is the mass, in grams, of 2.50 × 1022 atoms of Ca?" below is correct, except numbers in the last conversion factor have been replaced by the letters A and B. What are the numerical values of A and B, respectively?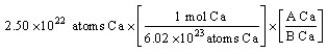
A) 1 mole and 6.02 x 1023 atoms
B) 40.08 g and 1 mol
C) 6.02 x 1023 g and 1 atom
D) 40.08 mol and 6.02 x 1023 atoms
Correct Answer:

Verified
Correct Answer:
Verified
Q8: Which of the following is the correct
Q9: Determine the empirical and molecular formulas for
Q10: 2.00 formula units of the compound K<sub>2</sub>S
Q11: How many moles of S are present
Q12: What is the formula weight of Ba(C<sub>2</sub>H<sub>3</sub>O<sub>2</sub>)<sub>2</sub>
Q14: Determine the percentage composition for the compound
Q15: One mole of OF<sub>2</sub> molecules contains _.<br>A)
Q16: Which of the following is the correct
Q17: Carbohydrates have an empirical formula of CH<sub>2</sub>O.
Q18: What is the molar mass of a