Multiple Choice
Which of the following is the correct "set-up" for the problem "How many moles of C are present in 25.00 grams of C6H12O6?"
A) 
B) 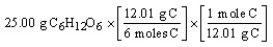
C) 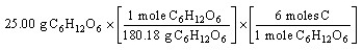
D) 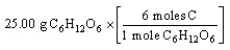
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: How many moles of S are present
Q12: What is the formula weight of Ba(C<sub>2</sub>H<sub>3</sub>O<sub>2</sub>)<sub>2</sub>
Q13: The "set-up" for the problem "What is
Q14: Determine the percentage composition for the compound
Q15: One mole of OF<sub>2</sub> molecules contains _.<br>A)
Q17: Carbohydrates have an empirical formula of CH<sub>2</sub>O.
Q18: What is the molar mass of a
Q19: Upon analysis, a compound having a molar
Q20: The "set-up" for the problem "How many
Q21: Match the statements on the left with