Multiple Choice
The "set-up" for the problem "How many oxygen atoms are there in 4.0 moles of N2O4?" below is correct, except for the use of letters rather than numbers in the conversion factors. What are the correct numerical values of A and D, respectively?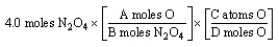
A) 4 and 4
B) 1 and 4
C) 4 and 1
D) 4 and 6.02 1023
Correct Answer:

Verified
Correct Answer:
Verified
Q15: One mole of OF<sub>2</sub> molecules contains _.<br>A)
Q16: Which of the following is the correct
Q17: Carbohydrates have an empirical formula of CH<sub>2</sub>O.
Q18: What is the molar mass of a
Q19: Upon analysis, a compound having a molar
Q21: Match the statements on the left with
Q22: Contrast the two members of each pair
Q23: Analysis of an unknown sample indicated the
Q24: Which of the following statements about "one-half
Q25: How many molecules of ethanol, C<sub>2</sub>H<sub>5</sub>OH, are