Essay
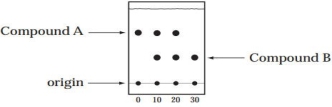
A student is following a reaction (the conversion of compound A to Compound B) by TLC. Aliquots of the reaction mixture are taken and analyzed at time = 0, 10, 20, and 30 min. The TLC plate, developed in 90% hexanes-10% ethyl acetate, is shown below.
(a) Which is the more polar compound, A or B?
(b) Is the reaction complete at 10 min? 20 min? 30 min?
(c) How can the identity of Compound B be verified by TLC?
Correct Answer:

Verified
(a) Compound B is the more polar compoun...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: 5 As a separation and detection method,
Q2: Consider the TLC plate illustrated in Problem
Q3: After a rather lengthy organic chemistry synthesis
Q4: A student spots an unknown sample on
Q5: If two compounds have R<sub>f </sub>values of
Q6: Calculate the R<sub>f </sub>values for the following
Q7: A wick of filter paper is placed
Q8: Again consider the TLC plate illustrated in
Q9: Consider a sample that is a mixture