Multiple Choice
Refer to the figure below, and then answer the question that follows.
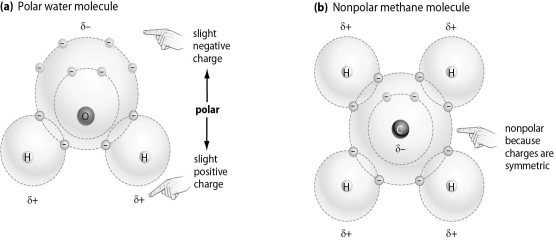
-Which of the following molecules is most likely to bind to an ion,and why?
A) Molecule A, because it has electrical charges that will attract an ion
B) Molecule B, because it has four hydrogen atoms on the exterior of the molecule
C) Molecule A, because any molecule with oxygen is able to bind to an ion
D) Molecule B, because it has a carbon at in the center of the molecule
Correct Answer:

Verified
Correct Answer:
Verified
Q50: Temperatures on the Earth are moderated by
Q51: All the mass of an atom is
Q52: Water molecules are uncharged and _.
Q53: Refer to the figure below, and then
Q54: Acids release hydrogen ions into aqueous solutions.
Q56: The naturally occurring helium atom is chemically
Q57: What are the three most important subatomic
Q58: The number of neutrons in the nucleus
Q59: As an acid mixes in water:<br>A)the number
Q60: For an atom to be considered an