Multiple Choice
Refer to the figure below, and then answer the question that follows.
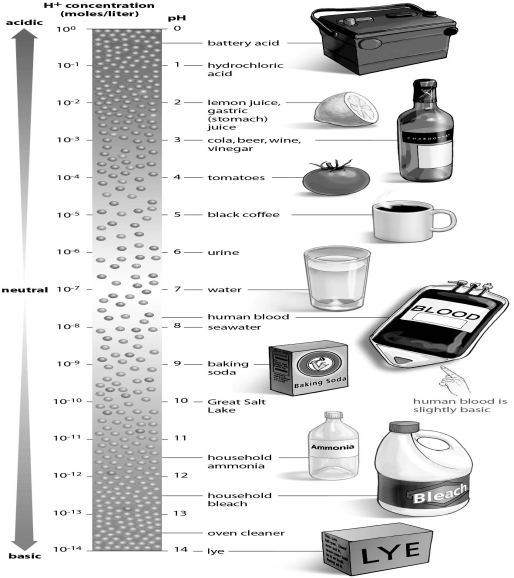
-You are working in a chemistry lab,and your lab partner knocks over a beaker of hydrochloric acid.You alert your laboratory instructor,and he immediately pours another solution over the spill to neutralize the acid.Using the figure as a guide,what did your instructor pour onto the acid to neutralize it?
A) water
B) baking soda
C) lemon juice
D) coffee
Correct Answer:

Verified
Correct Answer:
Verified
Q48: A(n)_ has a higher pH than a(n)_.
Q49: A polar covalent bond results when:<br>A)two atoms
Q50: Temperatures on the Earth are moderated by
Q51: All the mass of an atom is
Q52: Water molecules are uncharged and _.
Q54: Acids release hydrogen ions into aqueous solutions.
Q55: Refer to the figure below, and then
Q56: The naturally occurring helium atom is chemically
Q57: What are the three most important subatomic
Q58: The number of neutrons in the nucleus