Multiple Choice
On the energy diagram below,which arrow(s) represent the activation energy for the forward and reverse reactions? 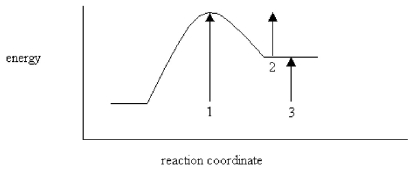
A) Arrow 1 is the activation energy for both the forward and reverse reactions.
B) Arrow 1 is the activation energy for the forward reaction and arrow 2 is the activation energy for the reverse reaction.
C) Arrow 1 is the activation energy for the forward reaction and arrow 3 is the activation energy for the reverse reaction.
D) Arrow 3 is the activation energy for the forward reaction and arrow 2 is the activation energy for the reverse reaction.
Correct Answer:

Verified
Correct Answer:
Verified
Q54: Match each of these terms with the
Q55: The active site of a certain enzyme
Q56: In the modified "lock-and-key" theory of enzyme
Q57: One reason the proximity effect enhances catalysis
Q58: In the nonpolar environment of most enzyme
Q60: Site directed mutagenesis is used to study
Q61: Most Km values of enzymes for their
Q62: Intermediates are more stable and have longer
Q63: The role of serine at the active
Q64: The induced fit model of enzyme activation