Multiple Choice
Use the following figure to answer the questions below.
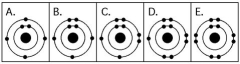
-Which drawing in the figure above depicts the electron configuration of an element with chemical properties most similar to Helium (2He) ?
A) A
B) B
C) C
D) D
E) E
Correct Answer:

Verified
Correct Answer:
Verified
Q4: How many electron pairs are shared between
Q8: What bonding or interaction is most likely
Q11: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5463/.jpg" alt=" -What results from
Q11: Which one of the atoms shown would
Q15: An atom has 6 electrons in its
Q17: An atom with atomic number 12 would
Q18: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5148/.jpg" alt=" -Refer to the
Q53: In ammonium chloride salt (NH₄Cl)the anion is
Q74: The nucleus of a nitrogen atom contains
Q75: What coefficients must be placed in the