Multiple Choice
Which one of the atoms shown would be most likely to form a cation with a charge of +1?
A) 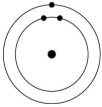
B) 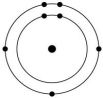
C) 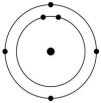
D) 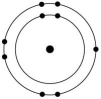
E) 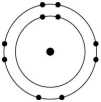
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: How many electron pairs are shared between
Q8: What bonding or interaction is most likely
Q11: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5463/.jpg" alt=" -What results from
Q15: Use the following figure to answer the
Q18: Which of the following statements is false?<br>A)
Q30: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5463/.jpg" alt=" -Which drawing in
Q36: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5463/.jpg" alt=" -In the figure
Q46: We can represent atoms by listing the
Q74: The nucleus of a nitrogen atom contains
Q75: What coefficients must be placed in the