Multiple Choice
Complete the following statement: The first law of thermodynamics states that
A) heat is a form of energy.
B) entropy is a function of state.
C) the entropy of the universe is increasing.
D) the change in the internal energy of a system is given by 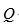 - W.
- W.
E) no engine can be more efficient than a Carnot engine operating between the same two temperatures.
Correct Answer:

Verified
Correct Answer:
Verified
Q30: A paddle wheel frictionally adds thermal energy
Q31: A container holding 1.2 kg of water
Q32: Enclosed beneath the moveable piston in the
Q33: In an isothermal process,1.59 moles of an
Q34: A heat engine operates between a hot
Q36: Beneath the moveable piston in the drawing,2.25
Q37: Which one of the following pressure-volume graphs
Q38: An ideal monatomic gas undergoes an adiabatic
Q39: A thermally isolated sample of an ideal
Q40: An ideal monatomic gas originally in state