Multiple Choice
Beneath the moveable piston in the drawing,2.25 moles of a monatomic ideal gas is enclosed at 314 K.The initial volume of the gas is 3.0 m³.The gas is compressed isothermally to a final volume of 1.0 m³.How much heat is removed from the gas during this process? 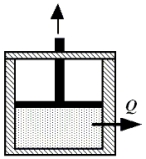
A) -6450 J
B) -4300 J
C) -2900 J
D) -1450 J
E) zero joules
Correct Answer:

Verified
Correct Answer:
Verified
Q31: A container holding 1.2 kg of water
Q32: Enclosed beneath the moveable piston in the
Q33: In an isothermal process,1.59 moles of an
Q34: A heat engine operates between a hot
Q35: Complete the following statement: The first law
Q37: Which one of the following pressure-volume graphs
Q38: An ideal monatomic gas undergoes an adiabatic
Q39: A thermally isolated sample of an ideal
Q40: An ideal monatomic gas originally in state
Q41: A system containing an ideal gas at