Multiple Choice
An ideal gas is taken from state A to state B through process shown on the pressure-volume graph.How much heat is added to the gas in this process? 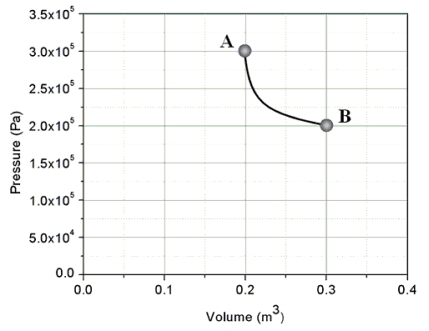
A) zero joules
B) 1.0 × 10⁴ J
C) 2.4 × 10⁴ J
D) 6.0 × 10⁴ J
E) This cannot be determined since n and T are not specified.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q44: A match ignites within in an oxygen-filled
Q45: Which one of the following statements is
Q46: Which one of the following statements is
Q47: An ideal monatomic gas expands isothermally from
Q48: An ideal monatomic gas expands isothermally from
Q50: A container holding 1.2 kg of water
Q51: A 1.00-kg sample of steam at 100.0
Q52: One mole of a monatomic gas at
Q53: Which one of the following statements best
Q54: Rick spends four hours researching on the