Multiple Choice
An ideal monatomic gas expands isothermally from state A to state B.The gas then cools at constant volume to state C.The gas is then compressed isobarically to D before it is heated until it returns to state A.
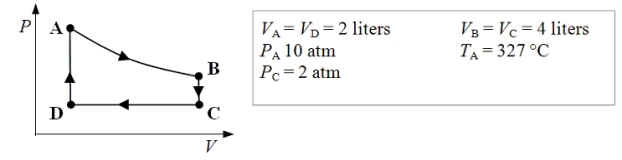
-What is the ratio of the internal energy of the gas in state C to that in state A?
A) 2/1
B) 1/2
C) 130/327
D) 240/600
E) 600/240
Correct Answer:

Verified
Correct Answer:
Verified
Q43: What are the SI units of the
Q44: A match ignites within in an oxygen-filled
Q45: Which one of the following statements is
Q46: Which one of the following statements is
Q47: An ideal monatomic gas expands isothermally from
Q49: An ideal gas is taken from state
Q50: A container holding 1.2 kg of water
Q51: A 1.00-kg sample of steam at 100.0
Q52: One mole of a monatomic gas at
Q53: Which one of the following statements best