Multiple Choice
An ideal monatomic gas expands isothermally from state A to state B.The gas then cools at constant volume to state C.The gas is then compressed isobarically to D before it is heated until it returns to state A.
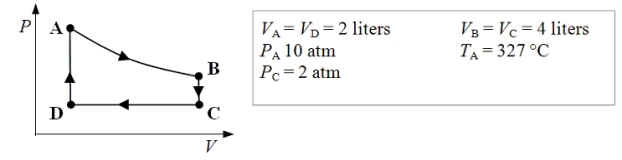
-What is the internal energy of the gas at point B?
A) 1 × 10³ J
B) 2 × 10³ J
C) 3 × 10³ J
D) 4 × 10³ J
E) 5 × 10³ J
Correct Answer:

Verified
Correct Answer:
Verified
Q1: In which one of these processes will
Q2: 5.00 kg of liquid water is heated
Q3: A block that slides on a rough
Q4: Two moles of an ideal gas have
Q6: A fixed amount of ideal gas is
Q7: During one stage of a reversible process,the
Q8: When the gas enclosed beneath the piston
Q9: Complete the following statement: Walls that separate
Q10: An ideal monatomic gas expands isobarically from
Q11: A container is divided into two chambers