Multiple Choice
A container is divided into two chambers that are separated by a valve.The left chamber contains one mole of a monatomic ideal gas.The right chamber is evacuated.At some instant,the valve is opened and the gas rushes freely into the right chamber.Which one of the following statements concerning this process is true? 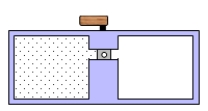
A) Work is done by the gas.
B) The temperature of the gas decreases.
C) The change in the entropy of the gas is zero.
D) The walls of the containing vessel must get colder.
E) The change in the internal energy of the gas is zero.
Correct Answer:

Verified
Correct Answer:
Verified
Q6: A fixed amount of ideal gas is
Q7: During one stage of a reversible process,the
Q8: When the gas enclosed beneath the piston
Q9: Complete the following statement: Walls that separate
Q10: An ideal monatomic gas expands isobarically from
Q12: An engine is used to lift a
Q13: An ideal monatomic gas originally in state
Q14: An ideal monatomic gas expands isothermally from
Q15: An isobaric process is represented on a
Q16: Neon is a monatomic gas with a