Short Answer
What is the concentration (M)of C 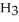 OH in a solution prepared by dissolving 3.96 g of C
OH in a solution prepared by dissolving 3.96 g of C  OH in sufficient water to give exactly 230 mL of solution?
OH in sufficient water to give exactly 230 mL of solution?
Correct Answer:

Verified
Correct Answer:
Verified
Q11: When 7.80 mL of 0.500 M AgNO<sub>3</sub>
Q12: Which of the following compounds will undergo
Q15: How many carbonate ions are present in
Q18: How many moles of Na I are
Q19: How many grams of AgNO<sub>3</sub> are needed
Q19: The chemical formula for sulfurous acid is<br>A)H<sub>2</sub>S(aq).<br>B)H<sub>2</sub>SO<sub>3</sub>(aq).<br>C)H<sub>2</sub>SO<sub>4</sub>(aq).<br>D)H<sub>2</sub>S<sub>2</sub>O<sub>7</sub>(aq).
Q20: A stock solution of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6105/.jpg" alt="A
Q33: A student prepared a stock solution by
Q133: If the reaction of phosphate ion with
Q204: Determine the concentration of a solution prepared