Multiple Choice
A stock solution of 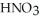 is prepared and found to contain 12.1 M of
is prepared and found to contain 12.1 M of 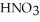 .If 25.0 mL of the stock solution is diluted to a final volume of 0.500 L,the concentration of the diluted solution is ________ M.
.If 25.0 mL of the stock solution is diluted to a final volume of 0.500 L,the concentration of the diluted solution is ________ M.
A) 0.242
B) 1.65
C) 0.605
D) 605
E) 242
Correct Answer:

Verified
Correct Answer:
Verified
Q15: How many carbonate ions are present in
Q16: What is the concentration (M)of C <img
Q18: How many moles of Na I are
Q19: How many grams of AgNO<sub>3</sub> are needed
Q19: The chemical formula for sulfurous acid is<br>A)H<sub>2</sub>S(aq).<br>B)H<sub>2</sub>SO<sub>3</sub>(aq).<br>C)H<sub>2</sub>SO<sub>4</sub>(aq).<br>D)H<sub>2</sub>S<sub>2</sub>O<sub>7</sub>(aq).
Q23: How many milliliters of a 0.266 M
Q25: An aqueous solution of H<sub> </sub>F is
Q125: How many grams of H<sub>2</sub> gas can
Q133: If the reaction of phosphate ion with
Q147: Give the net ionic equation for the