Multiple Choice
Lead ions can be precipitated from aqueous solutions by the addition of aqueous iodide: 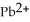 (aq) +
(aq) + 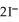 (aq) →
(aq) → 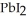 (s) Lead iodide is virtually insoluble in water so that the reaction appears to go to completion.How many milliliters of 3.550 M HI(aq) must be added to a solution containing 0.500 mol of
(s) Lead iodide is virtually insoluble in water so that the reaction appears to go to completion.How many milliliters of 3.550 M HI(aq) must be added to a solution containing 0.500 mol of 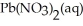 to completely precipitate the lead?
to completely precipitate the lead?
A) 3.55 × 10-3 mL
B) 282 mL
C) 141 mL
D) 0.141 mL
E) 0.282 mL
Correct Answer:

Verified
Correct Answer:
Verified
Q35: Choose the statement below that is TRUE.<br>A)
Q39: How many milliliters of 0.188 M <img
Q42: What is the concentration (M)of sodium ions
Q43: Identify Na Cl.<br>A) weak acid<br>B) weak electrolyte<br>C)
Q47: What volume (in mL)of 0.0887 M Mg
Q79: Define an electrolyte.
Q127: Calculate the concentration (M)of sodium ions in
Q196: What element is undergoing reduction (if any)in
Q213: Identify the spectator ions in the following
Q228: According to the following reaction,what mass of