Multiple Choice
Silver ions can be precipitated from aqueous solutions by the addition of aqueous chloride: 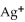 (aq) +
(aq) + 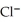 (aq) → AgCl(s) Silver chloride is virtually insoluble in water so that the reaction appears to go to completion.How many grams of solid NaCl must be added to 25.0 mL of 0.175 M
(aq) → AgCl(s) Silver chloride is virtually insoluble in water so that the reaction appears to go to completion.How many grams of solid NaCl must be added to 25.0 mL of 0.175 M 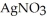 solution to completely precipitate the silver?
solution to completely precipitate the silver?
A) 4.38 × 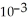 g
g
B) 7.49 × 10-5 g
C) 0.256 g
D) 0.0749 g
E) 1.34 × 104 g
Correct Answer:

Verified
Correct Answer:
Verified
Q83: Determine the name for aqueous HBr.<br>A)bromic acid<br>B)bromous
Q135: What is the oxidation number of the
Q140: Identify the oxidation state of Ca in
Q141: What is the molarity of a NaOH
Q142: Which of the following pairs of aqueous
Q142: If 100.mL of 0.200 M Na<sub>2</sub>SO<sub>4</sub> is
Q143: Identify K OH.<br>A) weak acid<br>B) weak electrolyte<br>C)
Q150: What element is undergoing oxidation (if any)in
Q177: In an acid-base neutralization reaction 23.74 mL
Q231: According to the following reaction,what volume of