Multiple Choice
What is the molarity of a NaOH solution if 39.1 mL of a 0.112 M 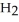
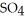 solution is required to neutralize a 25.0-mL sample of the NaOH solution?
solution is required to neutralize a 25.0-mL sample of the NaOH solution?
A) 0.350
B) 0.0716
C) 0.143
D) 54.7
E) 0.175
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q51: The titration of 25.0 mL of an
Q125: Based on the balanced chemical equation shown
Q135: What is the oxidation number of the
Q137: How many aluminum ions are present in
Q138: Which of the following is NOT a
Q140: Identify the oxidation state of Ca in
Q142: Which of the following pairs of aqueous
Q143: Identify K OH.<br>A) weak acid<br>B) weak electrolyte<br>C)
Q145: Silver ions can be precipitated from aqueous
Q177: In an acid-base neutralization reaction 23.74 mL