Multiple Choice
Calculate the pH for an aqueous solution of acetic acid that contains 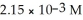 hydronium ion.
hydronium ion.
A) 4.65 × 10-12 M
B) 2.15 × 10-3 M
C) 2.67
D) 11.33
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q31: What is the hydronium ion concentration of
Q33: Which of the following is a strong
Q34: Which of the following is a Brønsted-Lowry
Q37: Calculate the pH of a 0.800 M
Q40: Which of the following is a polyprotic
Q47: What is the hydronium ion concentration of
Q63: What is the pH of a 0.100
Q72: Calculate the pH of a solution that
Q159: What is the K<sub>w</sub> of pure water
Q165: Which Br∅nsted-Lowry acid is not considered to