Multiple Choice
What is the hydronium ion concentration of a 0.400 M acetic acid solution with Ka = 1.8 × 10-5? The equation for the dissociation of acetic acid is: 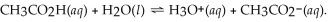
A) 2.7 × 10-2 M
B) 4.2 × 10-2 M
C) 2.7 × 10-3 M
D) 4.2 × 10-3 M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q29: An aqueous solution of ammonia is found
Q33: Which of the following is a strong
Q34: Which of the following is a Brønsted-Lowry
Q35: Calculate the pH for an aqueous solution
Q47: What is the hydronium ion concentration of
Q56: Calculate the hydroxide ion concentration in an
Q63: What is the pH of a 0.100
Q106: Describe the relationship between molecular structure and
Q148: Determine the [OH<sup>-</sup>] concentration of a 0.741
Q159: What is the K<sub>w</sub> of pure water