Multiple Choice
Calculate the pH for a buffer that is 0.10 M in NaO2CCO2Na and 0.50 M in NaO2CCO2H.K2 = 5.42 × 10−5, 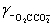 = 0.333,
= 0.333, 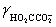 = 0.76
= 0.76
A) 4) 209
B) 4) 567
C) 3) 965
D) 4) 323
E) 3) 908
Correct Answer:

Verified
Correct Answer:
Verified
Q7: The rearrangement of the equilibrium constant expression
Q8: The mean fraction of protons, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4000/.jpg"
Q9: A solution is 0.120 M sodium oxalate
Q10: The equilibrium constants for a diprotic acid
Q11: A solution is 0.200 M in acetic
Q13: The pH of a solution composed of
Q14: Calculate the concentration of each fumaric acid,HO<sub>2</sub>CCHCHCO<sub>2</sub>H,species
Q15: Express [Ca<sup>2+</sup>] in terms [H<sup>+</sup>] for the
Q16: In addition to the equilibrium CaCO<sub>3</sub> ⇋
Q17: The mass balance equation for the solubility