Multiple Choice
Calculate the activity coefficient for phosphate in 0.0075 M KCl. 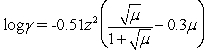
A) 0) 441
B) 0) 140
C) 0) 804
D) 0) 913
E) 0) 116
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: The charge balance equation for the solubility
Q2: Which is the correct effective equilibrium constant
Q3: Many ions do not have tabulated activity
Q4: A student is tasked to determine the
Q6: Calculate the pH of a sulfurous acid
Q7: The rearrangement of the equilibrium constant expression
Q8: The mean fraction of protons, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4000/.jpg"
Q9: A solution is 0.120 M sodium oxalate
Q10: The equilibrium constants for a diprotic acid
Q11: A solution is 0.200 M in acetic