For the Reaction Below,the Oxidizing Agent Is____________________ and It____________________ Electrons,and
Multiple Choice
For the reaction below,the oxidizing agent is____________________ and it____________________ electrons,and the reducing agent is____________________ and it____________________ electrons. 8 H+ + 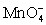 + 5 Cu+ ⇋ Mn2+ + 5 Cu2+ + 4 H2O
+ 5 Cu+ ⇋ Mn2+ + 5 Cu2+ + 4 H2O
A) 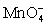
;gains 5;Cu+;loses 1
B) H+;loses 1;Cu+;gains 1
C) H+;gains 1;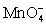
;loses 5
D) Cu+;loses 1;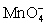
;gains 1
E) Cu+;loses 1;H+;gains 1
Correct Answer:

Verified
Correct Answer:
Verified
Q3: Which is true when electrochemical cells are
Q4: E<sup>o</sup><font face="symbol"></font> is defined as:<br>A)the formal half-cell
Q5: A galvanic cell is at equilibrium when:<br>A)E<sub>cell</sub>
Q6: All of the following are true for
Q7: For the line notation below,which is NOT
Q9: Calculate E<sup>°</sup><font face="symbol"></font> for the half-cell below.
Q10: Calculate the standard potential when the two
Q11: A galvanic cell with a large equilibrium
Q12: A cell is constructed according to the
Q13: Which is NOT correct for when the