Multiple Choice
A cell is constructed according to the cell diagram below.If the measured potential is 1.00 V,what is the pH of the dichromate cell? Fe|Fe2+ (aq,1M) || 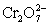 (aq 1 M) ,Cr2+ (aq,1 M) ,H+ (aq,x M) |Pt
(aq 1 M) ,Cr2+ (aq,1 M) ,H+ (aq,x M) |Pt 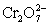 + 14 H+ + 6 e− ⇋ 2 Cr3+ + 7 H2O E° = 1.36 V
+ 14 H+ + 6 e− ⇋ 2 Cr3+ + 7 H2O E° = 1.36 V
Fe2+ + 2 e− ⇋ Fe (s) ____________________ E° = −0.44 V
A) 1) 931
B) 5) 797
C) 12.162
D) 6) 081
E) 4) 054
Correct Answer:

Verified
Correct Answer:
Verified
Q7: For the line notation below,which is NOT
Q8: For the reaction below,the oxidizing agent is_
Q9: Calculate E<sup>°</sup><font face="symbol"></font> for the half-cell below.
Q10: Calculate the standard potential when the two
Q11: A galvanic cell with a large equilibrium
Q13: Which is NOT correct for when the
Q14: Calculate the equilibrium constant for the reaction
Q15: Which of the following is NOT true
Q16: A bromide probe is constructed from a
Q17: Calculate the potential for the half-cell below