Short Answer
Calculate Eo and K for the cell composed of a bromate/bromide half-cell and a chlorate/chlorine dioxide half-cell. 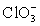 + 2 H+ + e− ⇋ ClO2 + H2O E° = 1.130 V
+ 2 H+ + e− ⇋ ClO2 + H2O E° = 1.130 V 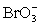 + 3 H2O + 6 e− ⇋ Br− + 6 OH− E° = 0.613 V
+ 3 H2O + 6 e− ⇋ Br− + 6 OH− E° = 0.613 V
Correct Answer:

Verified
E° = 0.517 ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q10: Calculate the standard potential when the two
Q11: A galvanic cell with a large equilibrium
Q12: A cell is constructed according to the
Q13: Which is NOT correct for when the
Q14: Calculate the equilibrium constant for the reaction
Q15: Which of the following is NOT true
Q16: A bromide probe is constructed from a
Q17: Calculate the potential for the half-cell below
Q19: The half-cells below are connected via a
Q20: For the silver half-reaction,Ag<sup>+</sup> + 1 e<sup>-</sup>