Multiple Choice
ΔH = +572 kJ for the decomposition of water by the reaction 2 H2O( 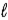 ) → 2 H2(g) + O2(g) .How many grams of water can be decomposed by 5.00 × 103 kJ of energy?
) → 2 H2(g) + O2(g) .How many grams of water can be decomposed by 5.00 × 103 kJ of energy?
A) 157 g
B) 315 g
C) 0.0629 g
D) 2.57 × 107 g
E) 5.15 × 107 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q32: A chemical reaction resulted in 1.376 g
Q33: In the combustion of natural gas according
Q34: Formation of two moles of hydrogen chloride
Q35: The reaction 2 Sb + 3 Cl<sub>2</sub>
Q36: Ammonium sulfate can be made by the
Q37: What is the theoretical yield of phosphorus
Q38: In the reaction CaCN<sub>2</sub> + 3 H<sub>2</sub>O
Q39: Silver nitrate and magnesium chloride solutions are
Q40: Which of the following best describes a
Q41: Cu<sub>2</sub>HgI<sub>4</sub> is prepared according to the equation