Multiple Choice
Consider the phase diagram shown.Choose the statement below that is true. 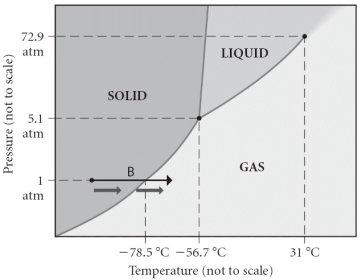
A) The triple point of this substance occurs at a temperature of 31°C.
B) At 10 atm of pressure,there is no temperature where the liquid phase of this substance would exist.
C) The solid phase of this substance is higher in density than the liquid phase.
D) The line separating the solid and liquid phases represents the ΔHvap.
E) None of the above are true.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Determine the vapor pressure (in mm Hg)of
Q93: Choose the pair of substances that are
Q94: Choose the substance with the highest vapor
Q95: Give the phase transition that occurs as
Q97: The enthalpy change for converting 10.0 g
Q99: Choose the substance with the lowest viscosity.<br>A)Cl<sub>3</sub>CCCl<sub>3</sub><br>B)Cl<sub>2</sub>CHCH<sub>2</sub>Cl<br>C)Cl<sub>2</sub>CHCHCl<sub>2</sub><br>D)ClCH<sub>2</sub>CH<sub>2</sub>Cl<br>E)Cl<sub>3</sub>CCHCl<sub>2</sub>
Q100: Which of the following compounds exhibits hydrogen
Q102: Identify the compound that does not have
Q103: Identify the term used to describe the
Q104: Which is expected to have the largest