Multiple Choice
The enthalpy change for converting 10.0 g of ice at -25.0°C to water at 80.0°C is ________ kJ.The specific heats of ice,water,and steam are 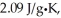
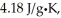 And
And 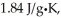 Respectively.For H2O,∆Hfus = 6.01 kJ/mol,and ∆Hvap = 40.67 kJ/mol.
Respectively.For H2O,∆Hfus = 6.01 kJ/mol,and ∆Hvap = 40.67 kJ/mol.
A) 12.28
B) 6.16
C) 3870
D) 7.21
E) 9.88
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Determine the vapor pressure (in mm Hg)of
Q92: Which of the following is considered a
Q93: Choose the pair of substances that are
Q94: Choose the substance with the highest vapor
Q95: Give the phase transition that occurs as
Q98: Consider the phase diagram shown.Choose the statement
Q99: Choose the substance with the lowest viscosity.<br>A)Cl<sub>3</sub>CCCl<sub>3</sub><br>B)Cl<sub>2</sub>CHCH<sub>2</sub>Cl<br>C)Cl<sub>2</sub>CHCHCl<sub>2</sub><br>D)ClCH<sub>2</sub>CH<sub>2</sub>Cl<br>E)Cl<sub>3</sub>CCHCl<sub>2</sub>
Q100: Which of the following compounds exhibits hydrogen
Q102: Identify the compound that does not have
Q104: Which is expected to have the largest