Multiple Choice
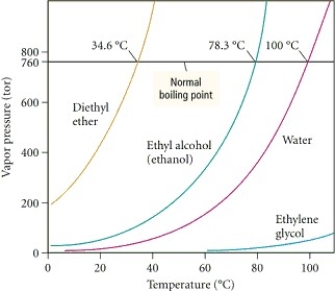
Based on the figure above,the boiling point of ethyl alcohol under an external pressure of 0.0724 atm is approximately ________°C.
A) 80
B) 60
C) 70
D) 40
E) 20
Correct Answer:

Verified
Correct Answer:
Verified
Q21: Ethanol (C<sub>2</sub>H<sub>5</sub>OH)melts at -114°C.The enthalpy of fusion
Q22: Which statement is correct?<br>A)The normal boiling point
Q23: Why is the ΔH<sub>vap</sub> higher than ΔH<sub>fus
Q24: Explain why the bolling point of water
Q25: Define sublimation.<br>A)The phase transition from solid to
Q27: Match the following.<br>-CH<sub>3</sub>CH<sub>3</sub><br>A)dipole-dipole forces<br>B)dispersion forces<br>C)ion-dipole forces<br>D)ionic bond<br>E)hydrogen
Q28: Identify the weakest type of intermolecular forces.<br>A)ion-dipole
Q29: Choose the substance with the highest boiling
Q30: The enthalpy change for converting 1.00 mol
Q31: Which of the following forms an ionic