Multiple Choice
The enthalpy change for converting 1.00 mol of ice at -50.0°C to water at 70.0°C is 
The specific heats of ice,water,and steam are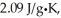
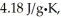
And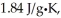
Respectively.For H2O,∆Hfus = 6.01 kJ/mol,and ∆Hvap = 40.67 kJ/mol.
A) 12.28
B) 6.41
C) 13.16
D) 7154
E) 9.40
Correct Answer:

Verified
Correct Answer:
Verified
Q25: Define sublimation.<br>A)The phase transition from solid to
Q26: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6106/.jpg" alt=" Based on the
Q27: Match the following.<br>-CH<sub>3</sub>CH<sub>3</sub><br>A)dipole-dipole forces<br>B)dispersion forces<br>C)ion-dipole forces<br>D)ionic bond<br>E)hydrogen
Q28: Identify the weakest type of intermolecular forces.<br>A)ion-dipole
Q29: Choose the substance with the highest boiling
Q31: Which of the following forms an ionic
Q32: Which of the following statements is not
Q33: In the phase diagram,what is the approximate
Q34: Identify the phase in which the water
Q73: Lithium crystallizes in a body-centered cubic structure.What