Multiple Choice
A 920-g empty iron kettle is put on a stove.How much heat in joules must it absorb to raise its temperature form 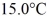
To 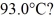
The specific heat for iron is 113 cal/kg ∙ °C,and 1 cal = 4.186 J.
A) 33,900 J
B) 40,500 J
C) 8,110 J
D) 40,100 J
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q65: For the mercury in a thermometer to
Q66: Oxygen condenses into a liquid at approximately
Q67: Consider a flat steel plate with a
Q68: A 6.5-g iron meteor hits the earth
Q69: A solid concrete wall has dimensions 4.0
Q71: The density of water at 0°C is
Q72: If,with steady state heat flow established,you double
Q73: The weather outside is frightful.The temperature is
Q74: A glass beaker of unknown mass contains
Q75: The coefficient of linear expansion for aluminum