Multiple Choice
A glass beaker of unknown mass contains 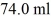
Of water.The system absorbs 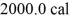
Of heat and the temperature rises 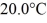
As a result.What is the mass of the beaker? The specific heat of glass is 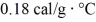
,and that of water is 1.0 cal/g ∙ °C.
A) 140 g
B) 560 g
C) 540 g
D) 270,000 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q69: A solid concrete wall has dimensions 4.0
Q70: A 920-g empty iron kettle is put
Q71: The density of water at 0°C is
Q72: If,with steady state heat flow established,you double
Q73: The weather outside is frightful.The temperature is
Q75: The coefficient of linear expansion for aluminum
Q76: A lab assistant pours 330 g of
Q77: The volume coefficient of thermal expansion for
Q78: In a flask,114.0 g of water is
Q79: Some properties of a certain glass are