Multiple Choice
A container of 114.0 g of water is heated using 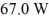
Of power,with perfect efficiency.How long will it take to raise the temperature of the water from 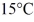
To 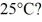
The specific heat capacity of the container is negligible,and the specific heat capacity of water is 4.186 × 103 J/kg ∙ C.
A) 71 s
B) 4.1 s
C) 17 s
D) 320,000 s
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: Nitrogen boils at -196°C.What is the corresponding
Q6: A glass flask has a volume of
Q7: A lab assistant drops a 400.0-g piece
Q8: If 150 kcal of heat raises the
Q9: Platinum melts at 3215°F.What is the corresponding
Q11: A concrete wall of a cold storage
Q12: In an electric furnace used for refining
Q13: On a cold day,a piece of metal
Q14: The temperature changes from 35°F during the
Q15: A person tries to heat up her