Multiple Choice
A lab assistant drops a 400.0-g piece of metal at 100.0°C into a 100.0-g aluminum cup containing 500.0 g of water at 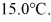
In a few minutes,she measures the final temperature of the system to be 40.0°C.What is the specific heat of the 400.0-g piece of metal,assuming that no significant heat is exchanged with the surroundings? The specific heat of this aluminum is 900.0 J/kg ∙ K and that of water is 4186 J/kg ∙ K.
A) 1900 J/kg ∙ K
B) 2270 J/kg ∙ K
C) 3300 J/kg ∙ K
D) 3800 J/kg ∙ K
E) 4280 J/kg ∙ K
Correct Answer:

Verified
Correct Answer:
Verified
Q2: A steel pipe 36.0 m long,installed when
Q3: The thermal conductivity of aluminum is twice
Q4: What is the net power radiated by
Q5: Nitrogen boils at -196°C.What is the corresponding
Q6: A glass flask has a volume of
Q8: If 150 kcal of heat raises the
Q9: Platinum melts at 3215°F.What is the corresponding
Q10: A container of 114.0 g of water
Q11: A concrete wall of a cold storage
Q12: In an electric furnace used for refining