Multiple Choice
A lab assistant pours 330 g of water at 45°C into an 855-g aluminum container that is at an initial temperature of 10°C.The specific heat of aluminum is 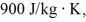
And that of water is 4186 J/kg ∙ K.What is the final temperature of the system,assuming no heat is exchanged with the surroundings?
A) 28°C
B) 32°C
C) 31°C
D) 33°C
E) 35°C
Correct Answer:

Verified
Correct Answer:
Verified
Q71: The density of water at 0°C is
Q72: If,with steady state heat flow established,you double
Q73: The weather outside is frightful.The temperature is
Q74: A glass beaker of unknown mass contains
Q75: The coefficient of linear expansion for aluminum
Q77: The volume coefficient of thermal expansion for
Q78: In a flask,114.0 g of water is
Q79: Some properties of a certain glass are
Q80: Which two temperature changes are equivalent?<br>A)1 K
Q81: The process in which heat flows by