Multiple Choice
Two containers of equal volume each hold samples of the same ideal gas.Container A has twice as many molecules as container B.If the gas pressure is the same in the two containers,the correct statement regarding the absolute temperatures TA and TB in containers A and B,respectively,is
A) TA = TB.
B) TA = 2TB.
C) TA =  TB.
TB.
D) TA = 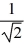 TB.
TB.
E) TA =  TB.
TB.
Correct Answer:

Verified
Correct Answer:
Verified
Q81: Consider two equal-volume flasks of gas at
Q82: The rms speed of a certain sample
Q83: The absolute temperature of a gas is
Q84: At what temperature is the rms speed
Q85: A sealed cylinder fitted with a movable
Q87: A brass wire 2.0 m long and
Q88: A sealed container holds 0.020 moles of
Q89: Oxygen molecules are 16 times more massive
Q90: A mole of diatomic oxygen molecules and
Q91: On a cold day,you take in 4.2