Multiple Choice
Consider two equal-volume flasks of gas at the same temperature and pressure.One gas,oxygen,has a molecular mass of 32.The other gas,nitrogen,has a molecular mass of 28.What is the ratio of the number of oxygen molecules to the number of nitrogen molecules in these flasks?
A) 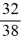
B) 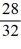
C) 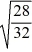
D) 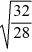
E) 
Correct Answer:

Verified
Correct Answer:
Verified
Q76: Dust particles in a grain elevator frequently
Q77: A quantity of an ideal gas is
Q78: A 360-g metal container,insulated on the outside,holds
Q79: Two experimental runs are performed to determine
Q80: A runner generates 1260 W of thermal
Q82: The rms speed of a certain sample
Q83: The absolute temperature of a gas is
Q84: At what temperature is the rms speed
Q85: A sealed cylinder fitted with a movable
Q86: Two containers of equal volume each hold