Multiple Choice
The root-mean-square speed of the molecules of an ideal gas is v.The gas is now slowly compressed to one-half its original volume with no change in temperature.What is the root-mean-square speed of the molecules now?
A) 4v
B) 2v
C) v/ 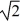
D) v
E) v/2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: A 45.0-kg sample of ice is at
Q20: A balloon originally has a volume of
Q21: The absolute temperature of an ideal gas
Q22: An ideal gas has a pressure of
Q23: A substance has a melting point of
Q25: If the temperature of an ideal gas
Q26: When a sample of water at 0.0°C
Q27: At what temperature would the root-mean-square speed
Q28: Solar houses use a variety of energy
Q29: A laboratory vacuum pump can reduce the