Multiple Choice
A substance has a melting point of 20°C and a heat of fusion of 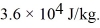
The boiling point is 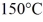
And the heat of vaporization is 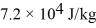
At a pressure of one atmosphere.The specific heats for the solid,liquid,and gaseous phases are 600 J/kg ∙ K (solid) ,1000 J/kg ∙ K (liquid) ,and 400 J/kg ∙ K (gaseous) .How much heat is given up by 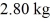
Of this substance when it is cooled from 170°C to 86°C at a pressure of one atmosphere?
A) 400 kJ
B) 200 kJ
C) 300 kJ
D) 440 kJ
E) 640 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q18: Two experimental runs are performed to determine
Q19: A 45.0-kg sample of ice is at
Q20: A balloon originally has a volume of
Q21: The absolute temperature of an ideal gas
Q22: An ideal gas has a pressure of
Q24: The root-mean-square speed of the molecules of
Q25: If the temperature of an ideal gas
Q26: When a sample of water at 0.0°C
Q27: At what temperature would the root-mean-square speed
Q28: Solar houses use a variety of energy