Multiple Choice
The temperature of an ideal gas in a sealed rigid 0.20- 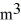
Container is reduced from 360 K to 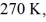
And the final pressure of the gas is 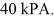
How much work is done by the gas during this process? (R = 8.31 J/mol ∙ K)
A) 0 kJ
B) -9.0 kJ
C) -12 kJ
D) 9.0 kJ
E) 12 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q55: An ideal gas is compressed isothermally to
Q56: The figure shows a pV diagram for
Q57: A certain ideal gas has a molar
Q58: An air conditioner with a coefficient of
Q59: If the efficiency of a Carnot engine
Q61: An ideal Carnot engine is operated as
Q62: A sealed 87- <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg" alt="A sealed
Q63: The figure shows a pV diagram for
Q64: How much heat is required to raise
Q65: Suppose that the Department of Energy develops