Multiple Choice
A compression at a constant pressure of 200 kPa is performed on 8.00 moles of an ideal monatomic gas.The compression reduces the volume of the gas from 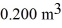
To 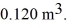
How much work was done by the gas during this process?
A) -16 kJ
B) 16 kJ
C) -40 kJ
D) 40 kJ
E) 0 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q29: A Carnot air conditioner has a coefficient
Q30: An external heat source supplies heat to
Q31: A nuclear power plant has an actual
Q32: An ideal gas undergoes an isothermal expansion.During
Q33: A gas expands from an initial volume
Q35: An ideal Carnot engine is operated as
Q36: Which one of the following is a
Q37: The work done on an ideal gas
Q38: Two processes are shown on the pV
Q39: An ideal Carnot engine extracts 529 J