Multiple Choice
Two processes are shown on the pV diagram in the figure.One of them is an adiabat and the other one is an isotherm.Which process is the isotherm? 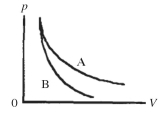
A) process A
B) process B
C) The processes shown are neither isotherms nor adiabats.
D) It is not possible to tell without knowing if the gas is monatomic or diatomic.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: A gas expands from an initial volume
Q34: A compression at a constant pressure of
Q35: An ideal Carnot engine is operated as
Q36: Which one of the following is a
Q37: The work done on an ideal gas
Q39: An ideal Carnot engine extracts 529 J
Q40: An ideal Carnot engine has an efficiency
Q41: For a certain ideal Carnot engine,the hot
Q42: An expansion process on an ideal diatomic
Q43: A certain ideal gas has a molar