Multiple Choice
An expansion process on an ideal diatomic ideal gas has a linear path between the initial and final coordinates on a pV diagram.The coordinates of the initial state are: the pressure is 300 kPa,the volume is 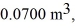
And the temperature is 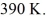
The final pressure is 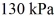
And the final temperature is 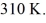
How much work is done by the gas during this process? (R = 8.31 J/mol ∙ K)
A) 13,000 J
B) 6,300 J
C) 9,400 J
D) 16,000 J
E) 19,000 J
Correct Answer:

Verified
Correct Answer:
Verified
Q37: The work done on an ideal gas
Q38: Two processes are shown on the pV
Q39: An ideal Carnot engine extracts 529 J
Q40: An ideal Carnot engine has an efficiency
Q41: For a certain ideal Carnot engine,the hot
Q43: A certain ideal gas has a molar
Q44: During an isothermal process,5.0 J of heat
Q45: A heat engine having the maximum possible
Q46: A sample of ideal monatomic gas is
Q47: A fluid in an insulated,flexible bottle is