Multiple Choice
The figure shows part of the energy level diagram of a certain atom.The energy spacing between levels 1 and 2 is twice that between 2 and 3.If an electron makes a transition from level 3 to level 2,the radiation of wavelength λ is emitted.What possible radiation wavelengths might be produced by other transitions between the three energy levels? 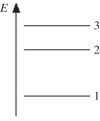
A) both λ/2 and λ/3
B) only λ/2
C) both 2λ and 3λ
D) only 2λ
Correct Answer:

Verified
Correct Answer:
Verified
Q6: The magnetic quantum number m<sub>1</sub> can have
Q7: In making a transition from state n
Q8: What is the value of n in
Q9: The only invalid electron state and shell
Q10: What is the wavelength of the photon
Q12: The shortest wavelength of a photon that
Q13: The elements in the periodic table that
Q14: The longest wavelength photon that can be
Q15: What is the electron configuration for Li,which
Q16: What is the greatest magnitude of the