Multiple Choice
The shortest wavelength of a photon that can be emitted by a hydrogen atom,for which the initial state is 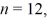
Is closest to which one of the following values? 
A) 92 nm
B) 82 nm
C) 72 nm
D) 62 nm
E) 52 nm
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: In making a transition from state n
Q8: What is the value of n in
Q9: The only invalid electron state and shell
Q10: What is the wavelength of the photon
Q11: The figure shows part of the energy
Q13: The elements in the periodic table that
Q14: The longest wavelength photon that can be
Q15: What is the electron configuration for Li,which
Q16: What is the greatest magnitude of the
Q17: Which of the following statements are true