Multiple Choice
Radium-226 decays into radon-222 plus an alpha particle. How much energy is released in this process? 1 u = 931.494 MeV/c2. The relevant mass values are: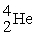 , 4.002603 u;
, 4.002603 u; 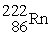 , 222.017570 u;
, 222.017570 u; 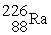 , 226.025402 u.
, 226.025402 u.
A) 4.24 MeV
B) 3.76 MeV
C) 4.87 MeV
D) 5.05 MeV
E) 5.39 MeV
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q32: Scientists hope that some day they will
Q33: If the "half life" for Radium-226 is
Q34: Plutonium-239 decays into uranium-235 plus an alpha
Q35: What is the missing term in this
Q38: If a 2.0-MeV neutron released in a
Q39: All nuclei of a given element have
Q40: A radioactive substance containing 4.00 × 10<sup>16</sup>
Q41: Photons associated with the magnetic field used
Q61: The number of radioactive nuclei in a
Q86: What is the approximate nuclear radius of