Multiple Choice
Plutonium-239 decays into uranium-235 plus an alpha particle. The energy released in the process is 5.24 MeV. Given the following mass values: 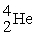 , 4.002603 u;
, 4.002603 u; 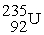 , 235.043924 u; what is the mass value of
, 235.043924 u; what is the mass value of 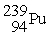 ?
?
1 u = 931.494 MeV/c2.
A) 239.052157 u
B) 239.027750 u
C) 239.001889 u
D) 238.9991889 u
E) 238.988842 u
Correct Answer:

Verified
Correct Answer:
Verified
Q29: The neutral deuterium atom, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6213/.jpg" alt="The
Q30: Complete the following nuclear decay equation:<br> <img
Q31: The density of a nucleus (to a
Q32: Scientists hope that some day they will
Q33: If the "half life" for Radium-226 is
Q35: What is the missing term in this
Q37: Radium-226 decays into radon-222 plus an alpha
Q38: If a 2.0-MeV neutron released in a
Q39: All nuclei of a given element have
Q61: The number of radioactive nuclei in a